
Feb 19, 2026 Biomedical Research and Testing Reimagined: Toward a Future Without Animal Suffering
Since December 2023, WBI has published six articles discussing the use of animals in biomedical research and testing. These articles comment on a variety of government, industry, and academic initiatives aimed at reducing animal use in laboratories by transitioning to NAMs (Non-Animal Methods or New Approach Methods).
In WBI’s November 2023 newsletter, the article Where is the Evidence that Laboratory Animal Use is Falling? included a graph (reproduced below) showing that laboratory animal use in five major industrial countries peaked in the mid-1970s. By 2019, animal use had decreased by 60-80% from its peak in these respective countries. However, readers are cautioned that estimating total laboratory animal use in any country is challenging, and early trends—except for Great Britain—are based on relatively few data points.
Nonetheless, trends in laboratory animal use across the five countries demonstrate a common pattern: growth until the 1970s, followed by a decline. Canada, however, did not experience a decline in laboratory animal use after the 1970s.
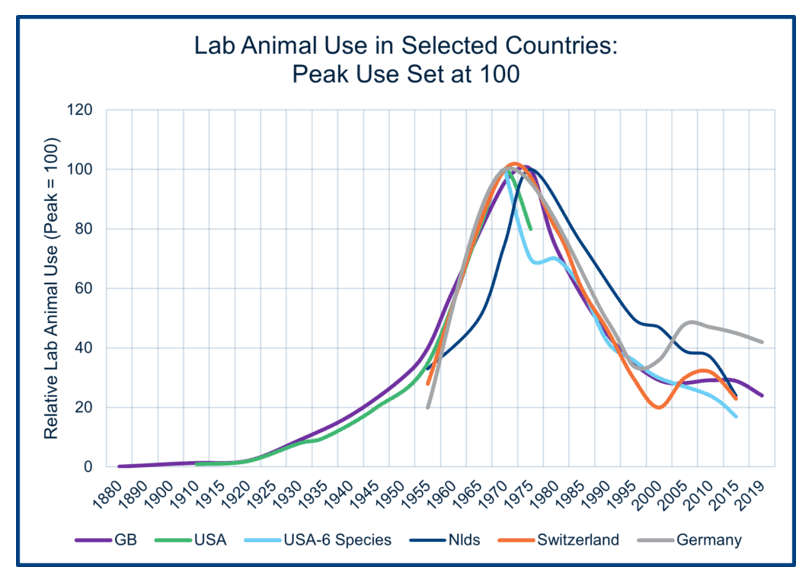
Data for the chart come from multiple sources, including surveys conducted in the mid-1950s by the International Committee for Laboratory Animal Science and national government reports for the USA, the Netherlands, Switzerland, Germany, and Great Britain.
The increases in laboratory animal use observed this century, as shown in the chart, are primarily due to the excitement surrounding the potential benefits of genetically modified mouse strains. However, these strains did not deliver the anticipated advancements in human health. A few years ago, two major mouse breeding facilities in Great Britain were shut down, and overall laboratory animal use is now declining again as the focus shifts toward NAMs.
On November 11, 2025, the UK Government announced plans to phase out the use of laboratory animals entirely. Instead, they will focus on new technologies that use human cell systems, which more accurately replicate human physiology and metabolism than do laboratory animals. The new strategy highlights the potential of organ-on-a-chip systems—small devices that use human cells to simulate the functions of human organs—as well as increased use of artificial intelligence (AI). AI will help analyze vast amounts of data on how molecules behave, enabling quicker, more reliable predictions of whether proposed new medicines will be safe and effective in humans.
The announced UK strategy sets specific targets to end regulatory animal testing for skin and eye irritation by the end of 2026. By 2027, the strategy aims to eliminate the use of mice in determining the safety of Botox preparations and to discontinue the General Safety Test, which detects viruses and bacteria that could inadvertently contaminate medicines. Science Minister Lord Vallance emphasized, “Nobody in our country of animal lovers wants to see suffering and our plan will support efforts to end animal testing whenever possible and roll out alternatives as soon as it is safe and effective to do so.” This strategy will be supported by £60 million in funding, a new data hub, and the establishment of a center designed to streamline the regulatory approval process for new alternative systems.
The reaction to Lord Vallance’s announced new strategy has been largely positive. Meanwhile, the USA was among the first to establish bold plans to reduce and, where appropriate and possible, end the use of laboratory animals.
Fifty years ago, the British NGO FRAME (the Fund for the Replacement of Animals in Medical Experiments) urged the British government to establish a center dedicated to developing alternatives to animal testing. This was in response to the Medical Research Council’s Laboratory Animals Centre located in southern England. Unfortunately, these appeals were largely ignored, although the Laboratory Animals Centre was closed in the early 1980s. In 2004, the National Centre for the 3Rs (NC3Rs) was established, and its director reports she is eager to contribute to the new strategy recently announced by Lord Vallance.
The European Union is working to keep pace with developments in the USA and the UK by creating its own Roadmap for the future of animal testing and new research methods. This roadmap is expected to be published in the first quarter of 2026.
Recent market reports indicate that the global market for NAMs is expected to grow significantly in the coming years. For instance, Global Market Insights estimates that the NAMs market will expand from $1.8 billion in 2023 to $4.4 billion by 2032, reflecting a Compound Annual Growth Rate (CAGR) of 11.9%. McKinsey highlights substantial growth in the NAMs sector, noting that “The shift away from animal testing is not just a response to ethical and regulatory pressures, it is also an opportunity to enhance the efficiency and predictivity of preclinical development.”
There is a growing consensus that animal testing has significant limitations in predicting human outcomes, and new technologies have emerged to address these challenges. A prominent indicator of changes in laboratory animal use is the recent announcement by Charles River Laboratories, the world’s largest breeder of laboratory animals, with approximately 35% of the global laboratory animal market share. In October 2025, Charles River announced the formation of a Scientific Advisory Board dedicated to advancing NAMs that could reduce reliance on animal testing in therapeutic development. The company has already integrated several NAMs into its drug discovery programs, including a bacterial endotoxin test, in vitro skin assays, and the use of virtual control groups.
Laboratory animal use in biomedical research has declined markedly in several industrialized countries since peaking in the 1970s, with recent momentum driven by the adoption of NAMs that more accurately reflect human biology. Governments, regulators, and scientific institutions are increasingly committing to transitioning away from animal testing, driven by technological innovation and targeted investment. For the many advocates who have worked tirelessly over decades to bring about this change, their goal is finally within reach: a future in which biomedical progress no longer depends on animal suffering.
Video credit: jitendrajadhav, iStock


