
Feb 03, 2023 Update – Food & Drug Administration’s Animal Testing Mandate
The bipartisan FDA Modernization Act of 2021 (S.5002) was introduced in the US Senate by Senator Cory Booker (D-N.J.) and Senator Rand Paul (R-Ky). Towards the end of 2022, the Act was modified and attached to the Omnibus Spending Bill. It was then signed into law by President Biden. Animal advocates have enthusiastically welcomed its passage, but it is unclear how much impact the Act will actually have in reducing animal use. Its central provision was the repeal of language in the 1938 Food Drug and Cosmetics Act that mandated animal testing before any new drug could be entered into human clinical trials. The updated language allows the FDA to use data from non-animal tests to qualify a new therapeutic for a human clinical trial.
The passage of the 1938 Act followed a public health scandal in which over 100 people died after taking an elixir of sulfanilamide that had been mistakenly prepared using a poisonous solvent. The mistake would likely have been detected if the mixture had been tested in animals before its distribution. It is, therefore, unsurprising that the 1938 Act included language requiring animal testing before a drug could be submitted to clinical trials.
After the Second World war, concern over unethical human experimentation reinforced the 1938 mandate regarding animal testing before human research. The Nuremberg trials included as defendants several medical professionals who had conducted research on human subjects in the concentration camps. The trials generated what has come to be known as the Nuremberg Code – consisting of ten points outlining the conditions for permissible medical experiments using human subjects. For example, the first point outlined the need for experimental subjects to give informed consent for participation in the experiment. The third point stated that any human experiment should be “based on the results of animal experimentation” and on an expectation that the results will justify the experiment.
Following the developments at the Nuremberg Code, the World Medical Association produced the first version of its Declaration of Helsinki in 1964. The Declaration consisted of 37 paragraphs outlining the conditions that should be met in any experimentation involving human subjects. The 21st paragraph in the 1964 Declaration was as follows:
“Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and adequate laboratory and, as appropriate, animal experimentation. The welfare of animals used for research must be respected.”
In other words, the requirement to conduct animal research before launching an experiment on humans became widely ingrained in biomedical research culture and set in stone via legislative language in the 1938 Act. The removal of the 1938 mandate should now be helpful to the FDA as the agency embraces using non-animal systems in developing and testing new drugs. For example, in a 2021 publication, the FDA Commissioner, Dr. Stephen Hahn, mentioned the robust support of the FDA “for the development of new regulatory tools that can help improve predictivity and potentially replace, reduce and/or refine animal testing.” These initiatives are driven by public pressures to reduce animal use and the exciting potential of new non-animal technologies that reduce the time and expense required to discover and develop new drugs while enhancing their safety and efficacy.
The data on laboratory animal use in the United States do not allow robust tracking of user trends. However, Great Britain’s Home Office has been collecting laboratory animal use data that extends back to the 19th century.
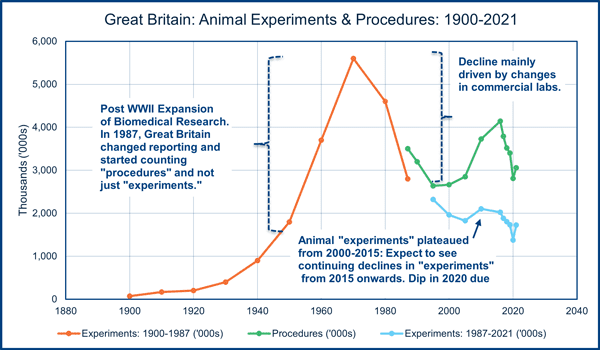 The British data shows a clear peak in laboratory animal use in the mid-1970s that has declined by around 70% since then. Similar downward trends from the mid-1970s can also be documented for the Netherlands and Switzerland. There is no doubt that the demand for laboratory animal use before human clinical trials is giving way to a range of sophisticated non-animal technologies that depend on cultured human cells and mimic the behavior of specific human organs. Dr. Francis Collins (the Director of the National Institutes of Health) predicted in testimony (at 33.47 of the video) before the US Senate in 2016 that the use of animals in drug development and safety testing would be mostly replaced by 2026. This replacement would be due to a move to the adoption of non-animal technologies “giving results that are more accurate, at lower cost and with higher throughput.”
The British data shows a clear peak in laboratory animal use in the mid-1970s that has declined by around 70% since then. Similar downward trends from the mid-1970s can also be documented for the Netherlands and Switzerland. There is no doubt that the demand for laboratory animal use before human clinical trials is giving way to a range of sophisticated non-animal technologies that depend on cultured human cells and mimic the behavior of specific human organs. Dr. Francis Collins (the Director of the National Institutes of Health) predicted in testimony (at 33.47 of the video) before the US Senate in 2016 that the use of animals in drug development and safety testing would be mostly replaced by 2026. This replacement would be due to a move to the adoption of non-animal technologies “giving results that are more accurate, at lower cost and with higher throughput.”


